kerosene vs diesel: Applications, Properties, and Safety
Diesel, Kerosene, Petroleum ProductsIn the realm of petroleum products, kerosene and diesel fuel (also known as gas oil) stand out as two crucial and widely used commodities. Both are hydrocarbon liquids derived from the distillation of crude oil. While they share a common origin, significant structural and chemical distinctions lead to diverse applications and unique characteristics. Understanding these differences is paramount for proper fuel selection, adherence to safety protocols, and appreciating their environmental implications.
This comprehensive article meticulously explores the distinctions between kerosene and diesel fuel, covering their chemical composition, physical properties, common applications, safety considerations, and environmental impact.
1. Chemical Composition and the Distillation Process
The most fundamental differences between kerosene and diesel fuel lie in their chemical makeup and boiling points. Both are composed of hydrocarbons (compounds of carbon and hydrogen), but the specific ratio and types of these hydrocarbons vary.
Kerosene (Paraffin)
Kerosene is obtained at a lower temperature range during the crude oil distillation process. It primarily consists of lighter hydrocarbons with shorter carbon chains, typically containing between 10 and 15 carbon atoms. As a result:
- It has a comparatively lower boiling point (approximately 150 to 280°C).
- It is more volatile and tends to evaporate more readily.
Diesel Fuel (Gas Oil)
Diesel fuel is separated at a higher temperature range than kerosene, but below that of lubricating oils, during distillation. This fuel contains heavier hydrocarbons with longer carbon chains, usually ranging from 12 to 20 carbon atoms. Consequently:
- It possesses a higher boiling point (approximately 180 to 370°C).
- It exhibits lower volatility.
2. Key Physical and Chemical Properties
The differing chemical compositions impart distinct physical and chemical properties to kerosene and diesel fuel.
Density
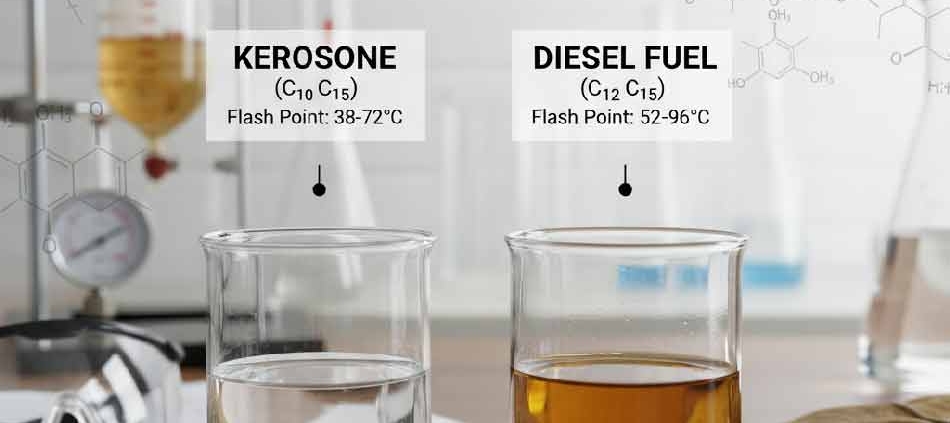
- Kerosene: Has a lower density (around 0.78 to 0.81 grams per cubic centimeter).
- Diesel Fuel: Has a higher density (approximately 0.82 to 0.86 grams per cubic centimeter). This density difference influences the energy stored per unit volume and the fuel’s behavior within pumps and injectors.
Flash Point
The flash point is the minimum temperature at which a fuel produces enough vapor to form an ignitable mixture with air. This property is vital for safety:
- Kerosene: Features a lower flash point (typically 38 to 72°C). This means it can release flammable vapors at relatively lower temperatures.
- Diesel Fuel: Has a higher flash point (usually 52 to 96°C). This characteristic makes diesel fuel safer at normal ambient temperatures and less prone to ignition.
Viscosity
- Kerosene: Has lower viscosity, making it more fluid.
- Diesel Fuel: Exhibits higher viscosity, which is crucial for lubricating the moving parts within diesel engine fuel delivery systems.
Cloud Point and Pour Point
These points describe a fuel’s behavior at low temperatures:
- Kerosene: Thanks to its lighter hydrocarbons, it remains fluid at significantly lower temperatures, demonstrating better cold resistance.
- Diesel Fuel: At lower temperatures, due to the presence of paraffins, it may become cloudy (cloud point) and then solidify into a gel (pour point). This can lead to filter clogging and engine issues in diesel vehicles. Special winter-grade diesel fuels are therefore produced for cold climates.
Sulfur Content
Historically, both fuels contained higher levels of sulfur. However, stringent environmental regulations have drastically reduced sulfur content in both, particularly in ultra-low sulfur diesel (ULSD). Sulfur contributes to sulfur oxides (SOx), which are major air pollutants and a cause of acid rain.
Cetane Number
- Diesel Fuel: This is a crucial indicator for diesel fuel. The cetane number reflects the fuel’s combustion quality in diesel engines; a higher cetane number signifies quicker and more complete ignition.
- Kerosene: A cetane number is not typically defined for kerosene, as it is not designed for diesel engines.
Odor and Color
- Kerosene: Usually has a distinct odor and, when pure, is colorless or slightly yellow.
- Diesel Fuel: Possesses a stronger, more characteristic odor and is generally yellow or green (in some countries, color is used to distinguish different grades).
3. Applications
The variations in chemical and physical properties dictate the primary applications of kerosene and diesel fuel.
Kerosene Applications
- Jet Fuel (Kerosene-type Jet Fuel): The most common and vital use of kerosene is as jet fuel. Its low freezing point and high energy density per unit volume make it ideal for jet engines.
- Lamp and Heater Fuel: In the past, and still in some rural areas or developing countries, kerosene serves as fuel for lamps and kerosene heaters (e.g., primus stoves).
- Industrial Solvent and Thinner: Due to its solvency, it is used as a solvent and thinner (for products like paints) in various industrial processes.
- Cleaning Agent: It is sometimes employed for cleaning and degreasing tasks.
Diesel Fuel Applications
- Diesel Engine Fuel: The primary and most widespread application of diesel fuel is as the fuel for various diesel engines. These engines power:
- Heavy vehicles: Trucks, buses, trains, ships.
- Agricultural machinery: Tractors, combine harvesters.
- Construction equipment: Loaders, bulldozers.
- Power generators: For emergency or continuous electricity generation.
- Some passenger cars: Diesel-powered automobiles.
- Heating Oil: In certain countries, a specific type of diesel fuel is used for domestic and industrial heating systems.
4. Safety and Storage
The differing flash points significantly impact the safety precautions required for storing and handling these two products.
- Kerosene: Given its lower flash point, it’s more volatile, and its vapors can be ignitable at room temperature. Therefore, storing it in enclosed, poorly ventilated spaces is riskier. It should always be kept in sealed containers in a cool, well-ventilated area.
- Diesel Fuel: With its higher flash point, it is safer at room temperature and doesn’t readily produce ignitable vapors. Nevertheless, it must still be handled with caution and stored according to safety guidelines.
For both fuels, the use of appropriate containers, adequate ventilation, keeping them away from heat sources and sparks, and training personnel involved in handling them are absolutely essential.
5. Environmental Aspects and Pollution
The combustion of both fuels results in the production of greenhouse gases (carbon dioxide), but there are differences in other pollutants.
- Diesel Fuel: Due to its heavier hydrocarbons and the combustion process in diesel engines, it may produce more particulate matter (soot) and nitrogen oxides (NOx). However, recent advancements in diesel engine technology and the widespread use of ultra-low sulfur diesel (ULSD) have substantially reduced these emissions.
- Kerosene: Produces less particulate matter pollution but still generates greenhouse gases.
Global initiatives are continuously working to reduce sulfur in fuels to prevent acid rain and associated respiratory issues.
6. Comprehensive Comparison: Kerosene vs. Diesel Fuel
To summarize and provide a clearer understanding of their differences, the following table compares the key aspects of kerosene and diesel fuel:
| Feature / Aspect | Kerosene (Paraffin) | Diesel Fuel (Gas Oil) |
|---|---|---|
| Distillation Origin | Lighter fraction from crude oil | Heavier fraction from crude oil |
| Carbon Chain | C10-C15 (Lighter) | C12-C20 (Heavier) |
| Boiling Point | 150-280°C (Lower) | 180-370°C (Higher) |
| Density | 0.78-0.81 g/cm³ (Lower) | 0.82-0.86 g/cm³ (Higher) |
| Flash Point | 38-72°C (Lower, more volatile) | 52-96°C (Higher, safer) |
| Viscosity | Lower (more fluid) | Higher |
| Cloud/Pour Point | Better cold resistance | Prone to clouding/gelling in cold (without additives) |
| Cetane Number | Not defined | Important for ignition quality (45-55) |
| Primary Applications | Jet fuel, lamps and heaters, industrial solvent | Diesel engines (heavy vehicles, generators, machinery) |
| Pollution | Lower particulate matter | Potentially more particulate matter and NOx (reduced with ULSD) |
| Odor | Distinct | Stronger, more characteristic |
Conclusion
Despite both originating from crude oil, kerosene and diesel fuel possess fundamental differences in their chemical composition, physical properties, and intended uses. While kerosene, with its higher volatility and lower flash point, is primarily recognized as jet fuel and an industrial solvent, diesel fuel, with its higher density, viscosity, and safer flash point, serves as the main fuel for diesel engines and heavy machinery.
Selecting the appropriate product not only influences system performance but also plays a critical role in ensuring safety and mitigating environmental impact. We hope this comprehensive guide has provided you with a deeper understanding of the distinctions between these two valuable petroleum products.










What is the main difference in chemical composition and distillation process between kerosene and diesel?
Thank you for your question! Kerosene is obtained from a lower temperature range in the crude oil distillation tower and is primarily composed of lighter hydrocarbons with shorter carbon chains (C10 to C15). Consequently, it has a lower boiling point (150 to 280°C) and is more volatile.
In contrast, diesel is separated at a higher temperature range than kerosene and consists of heavier hydrocarbons with longer carbon chains (C12 to C20). This gives it a higher boiling point (180 to 370°C) and lower volatility. This difference in hydrocarbon structure distinguishes their physical properties and applications.
The article says Kerosene is used as Jet Fuel. Why is it chosen over regular Diesel for aircraft? Is it just the lower freeze point, or is there a specific energy content advantage?
The primary reason is the low freeze point (or pour point), which is crucial for high-altitude operations where temperatures drop below $-40^\circ \text{C}$. Additionally, although Kerosene has a lower energy density by weight than Gasoline, its higher density by volume gives it a higher energy content per unit volume than Diesel, which is essential for maximizing range with limited tank space.
Why is storing kerosene riskier than diesel in warm environments
Because kerosene has a lower “flash point.” This means it produces flammable vapors at lower temperatures. In warm environments, these vapors accumulate faster, and even a small spark can cause a fire, whereas diesel is much safer at normal temperatures.