Introduction to Naphtha Types and Their Applications
Naphtha, often referred to as light gasoline or petroleum ether, is a liquid hydrocarbon fraction derived from the fractional distillation of crude oil in refinery units. Due to its unique physical and chemical properties, it finds extensive use as a feedstock and solvent in various industries.
In the crude oil refining process, which involves separating hydrocarbons based on their boiling points, naphtha is extracted as an intermediate cut between light gases and kerosene. Besides crude oil, naphtha can also be produced from other sources such as natural gas condensates and products from the distillation of coal tar. In the specialized terminology of petroleum engineering, the naphtha cut typically boils in the temperature range of 30 to 200 degrees Celsius and comprises hydrocarbons with 5 to 12 carbon atoms in their molecular structure. By weight, naphtha usually constitutes 15 to 30 percent of the crude oil composition and serves as a key intermediate in refining processes for the production of higher value-added products.
The Significance of Naphtha in the Oil and Petrochemical Industry
Naphtha plays a fundamental role as a primary feedstock in the petrochemical industry. This hydrocarbon fraction is used as feed for units producing a variety of chemicals, including ethylene, propylene, and butadiene. These light olefins are the basic building blocks in the production of polymers, elastomers, and other essential petrochemical materials. Additionally, naphtha is used as an industrial solvent and as a component in some gasoline formulations.
In key refinery units such as steam cracking, catalytic reforming, and isomerization units, naphtha is processed as the main feed and converted into higher-value products like high-octane gasoline and petrochemical intermediates. Therefore, naphtha is not merely a byproduct but a vital structural component in the production of a wide range of essential materials that impact various industries, from transportation to manufacturing.
The Production Process of Naphtha from Crude Oil
Crude oil is a complex mixture of various hydrocarbons with different molecular structures. The refining process, primarily carried out through fractional distillation, allows for the separation of these compounds based on the differences in their boiling points. In an atmospheric distillation tower, crude oil, after preheating to about 350 degrees Celsius, enters the tower, and its various components vaporize. Lighter compounds ascend to the top of the tower and condense at higher levels, while heavier components remain at the bottom.
The naphtha cut in this distillation process, as an intermediate component, is extracted between lighter cuts (such as liquefied petroleum gas) and heavier cuts (such as kerosene) in the distillation tower. This position in the tower determines a specific boiling point range for naphtha. Typically, naphtha is obtained as a liquid from the upper part of the crude oil distillation unit and is known as virgin naphtha or straight-run naphtha. This is the primary source of naphtha in most refineries, constituting about 11 percent of the input crude oil volume.
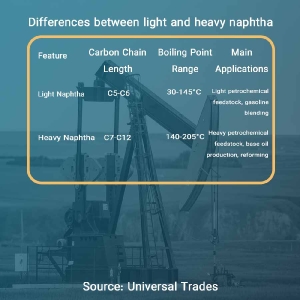
Main Classifications of Naphtha: Light Naphtha and Heavy Naphtha
Naphtha can be divided into two main categories based on its boiling point range and the carbon chain length of the constituent hydrocarbons: light naphtha and heavy naphtha.
Light Naphtha:
- Characteristics: Primarily composed of hydrocarbon molecules with 5 to 6 carbon atoms, resulting in a lower boiling point.
- Chemical Composition: Rich in paraffinic hydrocarbons with straight-chain or branched structures.
- Boiling Point Range: Typically boils in the temperature range of 30 to 145 degrees Celsius.
- Common Applications:
- As feedstock in petrochemical units for the production of light olefins such as ethylene and propylene.
- As a solvent in the paint, coating, and printing industries.
- As a component of gasoline and an additive to enhance its octane number.
- As feedstock for isomerization units to produce high-octane gasoline.
- To reduce the viscosity of heavy crude oil to facilitate the transfer process.
Heavy Naphtha:
- Characteristics: Primarily composed of hydrocarbon molecules with 7 to 12 carbon atoms, resulting in a higher boiling point.
- Chemical Composition: Rich in naphthenic hydrocarbons with saturated cyclic structures.
- Boiling Point Range: Typically boils in the temperature range of 140 to 205 degrees Celsius.
- Common Applications:
- As feedstock in units producing base lubricating oils and asphalt.
- As the main feedstock for catalytic reforming units to produce high-octane gasoline components such as aromatics.
- To produce low-sulfur naphtha cuts for gasoline and jet fuel production.
Other Classifications of Naphtha:
In addition to the main classification based on molecular weight and boiling point, naphtha can also be categorized based on its dominant chemical composition (paraffinic, naphthenic, aromatic) and its end-use (such as solvent naphthas with specific boiling point ranges). Industrial standards such as API and ASTM have also defined numerous specifications and test methods for different types of naphtha, ensuring the quality and consistency of this product in trade and industry.
Diverse Applications of Naphtha in Various Industries:
- Petrochemical Industry: Naphtha is used as the main feedstock in steam cracking units to produce ethylene and propylene, which are raw materials for the production of polymers, synthetic fibers, and other chemical materials.
- Fuel Industry: Naphtha is used as feedstock for catalytic reforming units to produce high-octane gasoline and as a blending component in the formulation of gasoline and jet fuel.
- Solvent Industry: Various types of naphtha with different boiling point ranges are used as industrial solvents in the production of paints, coatings, adhesives, and cleaning agents. VM&P (Varnish Makers’ and Painters’) naphtha is an example of this category.
- Other Applications: In specific applications, naphtha is used as fuel in camping stoves and lanterns (referred to as “white gas”), lighter fluid, and as a diluent in the tar sands mining industry.
The Relationship Between Naphtha and Other Petroleum Products:
In the production chain of petroleum products, naphtha occupies a position between lighter cuts such as liquefied petroleum gas and heavier cuts such as kerosene and diesel fuel. In the refining process, naphtha is extracted as an intermediate component and then undergoes various conversion processes based on its type and end-use, becoming products such as gasoline, solvents, and petrochemical feedstock. In contrast, heavier products like bitumen and base oils are obtained from heavier crude oil cuts after the removal of naphtha, although in some cases, naphtha can also act as a solvent or raw material in the production of these products.
Conclusion: The Strategic Importance of Naphtha
In conclusion, naphtha, as a versatile hydrocarbon fraction, plays a significant role in the oil, petrochemical, and other related industries. Its unique physical and chemical properties allow it to be used as a raw material, solvent, and component of various fuels. Therefore, naphtha is considered a vital intermediate in the value chain of the oil and chemical industries, and its stable supply is of strategic importance.











What is Naphtha and what is its position in the crude oil refining process? Also, what are the main differences between light naphtha and heavy naphtha?
Thank you for your careful reading of the article. Here is the answer to your question:
Naphtha, often known as light gasoline or petroleum solvent, is a liquid hydrocarbon cut obtained from the fractional distillation of crude oil in refineries. This substance typically boils in the temperature range of 30 to 200 degrees Celsius and consists of hydrocarbons with 5 to 12 carbon atoms.
Naphtha’s Position in the Crude Oil Refining Process:
In the atmospheric distillation process of crude oil, naphtha is recovered as an intermediate cut between lighter gases (like LPG) and heavier cuts (such as kerosene and diesel) in the distillation tower. This position means that naphtha typically constitutes about 15 to 30 percent of the crude oil composition and serves as a key intermediate material for producing higher value-added products in the petrochemical and fuel industries.
Main Differences Between Light Naphtha and Heavy Naphtha:
Light Naphtha:
Characteristics: Consists of hydrocarbons with 5 to 6 carbon atoms and has a lower boiling point (typically 30 to 145 degrees Celsius).
Chemical Composition: Rich in paraffinic hydrocarbons (straight or branched chain structures).
Applications: Primarily used as feedstock in petrochemical units for producing light olefins like ethylene and propylene, as a solvent in the paint and printing industries, a blending component in gasoline, and feedstock for isomerization units.
Heavy Naphtha:
Characteristics: Consists of hydrocarbons with 7 to 12 carbon atoms and has a higher boiling point (typically 140 to 205 degrees Celsius).
Chemical Composition: Rich in naphthenic hydrocarbons (saturated cyclic structures).
Applications: Primarily used as main feedstock for catalytic reforming units to produce high-octane gasoline components (like aromatics), and for producing low-sulfur petroleum cuts for jet fuel.
Since Naphtha is an intermediate cut, how significant is the price difference between Light Naphtha (used for petchem feed) and Heavy Naphtha (used for gasoline reforming)? Does one trade more actively than the other?
Light Naphtha generally commands a premium because it’s the primary feedstock for steam crackers (producing high-value ethylene and propylene). Its price often correlates more closely with petrochemical demand than crude oil or gasoline. Heavy Naphtha pricing is typically tied more to gasoline margins and the economics of the catalytic reformer.
Отличная статья, очень подробно освещает типы и применение нафты в промышленности! У меня возник вопрос по медицинскому применению производных нефти. В тексте упоминается использование нафты как растворителя, но я также встречал информацию о ее применении в медицине, в частности, о препарате под названием “Нафталан”. Он, если я не ошибаюсь, производится из очищенной нефти и используется для лечения кожных заболеваний. Не могли бы вы подробнее рассказать, является ли этот медицинский препарат производным от одного из описанных вами типов нафты (легкой или тяжелой) и какой процесс очистки для этого требуется? Для справки, я нашел описание этого препарата здесь: . Буду очень признателен за разъяснение этого момента, так как это соединяет технические аспекты с практическим применением в совершенно другой области.
Нафталан производится из Нафталанской нефти — уникального вида сырой нефти, который добывается исключительно в регионе Нафталан в Азербайджане.
Everyone loves what you guys are usually up too.
This type of clever work and exposure! Keep up
the very good works guys I’ve added you guys to our blogroll.