Kerosene Production Process: From Crude Oil to Home and Industrial Use
Kerosene, once a primary fuel for home heating and lighting not so long ago, is still used today in various industries, jet engines, and even in specific heating applications, despite its reduced household consumption. But how is this useful substance extracted from the earth and transformed into a usable product? This article delves into the kerosene production process from start to finish.
What is Kerosene?
Kerosene is a petroleum product obtained from the distillation of crude oil. It’s a clear, colorless (or slightly yellowish) liquid with a distinct odor. Kerosene’s boiling point ranges between 150 to 300 degrees Celsius, placing it in the category of mid-range crude oil fractions.
Main Stages of Kerosene Production
Kerosene production involves several key stages, all carried out in oil refineries:
1. Crude Oil Extraction
The first step in this process is extracting crude oil from underground reservoirs. After exploration and drilling, crude oil is brought to the surface by special pumps and transported to refineries.
2. Crude Oil Preparation (Desalting and Dehydration)
Extracted crude oil usually contains water, salt, and suspended particles. To prevent corrosion of refinery equipment and improve product quality, these impurities must be removed. This is done in the desalting and dehydration unit.
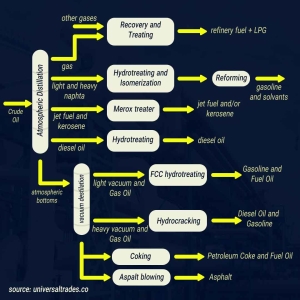
3. Atmospheric Distillation
The key stage in kerosene production is atmospheric distillation. Preheated crude oil enters the atmospheric distillation tower. In this tower, crude oil is heated to about 350 to 400 degrees Celsius. Due to this heat, different crude oil components vaporize based on their varying boiling points and condense at different heights within the distillation tower.
Kerosene is collected in the middle cut of this tower. This means lighter components (like petroleum gases and gasoline) rise to the top of the tower, while heavier components (like diesel and fuel oil) settle at the bottom.
4. Purification and Quality Improvement (Hydrotreating)
Kerosene exiting directly from the atmospheric distillation tower may contain impurities such as sulfur compounds. These compounds can cause pollution and corrosion. Therefore, the produced kerosene undergoes a process called hydrotreating.
In hydrotreating, kerosene reacts with hydrogen under high pressure and temperature in the presence of a catalyst. This reaction converts sulfur compounds into hydrogen sulfide (H2S), which is then removed. This stage improves the quality of kerosene in terms of color, odor, and stability, preparing it for various uses.
5. Storage and Distribution
After purification, kerosene is transferred to storage tanks. From there, the product is transported via pipelines, tankers, or ships to distribution centers and end-users.
Applications of Kerosene
Given its properties, kerosene has diverse applications, including:
- Jet engine fuel: The most important modern use of kerosene is as Jet Fuel.
- Fuel for kerosene lamps and lanterns: Very common in the past and still used in specific areas.
- Heating fuel: In kerosene heaters and specific heating systems.
- Solvent and cleaner: Used as a solvent and cleaner in some industries.
- Pesticides: As a component in certain agricultural pesticide formulations.
- Lighting in remote areas: In places without access to electricity.
Conclusion
As we’ve seen, kerosene production is a complex, multi-stage process that begins with extracting crude oil from deep within the earth. After undergoing preparation, distillation, and purification stages in refineries, it transforms into a high-quality final product with diverse applications. From jet engine fuel to heating and industrial uses, kerosene continues to hold a special place in the world’s energy and chemical basket, shaping a significant part of our daily lives. Understanding this process not only helps us better appreciate the importance of this substance but also provides an overview of the complexities and technologies involved in the oil and gas industry.
Frequently Asked Questions (FAQ)
1. What exactly is kerosene?
Kerosene is a petroleum product obtained from the distillation of crude oil. It is a clear or slightly yellowish liquid with a distinct odor, and its boiling point ranges from 150 to 300 degrees Celsius.
2. What is the main difference between kerosene, gasoline, and diesel?
The main difference lies in their boiling points and chemical composition. Gasoline has a lower boiling point (30 to 200 degrees Celsius) and is lighter, while diesel (gas oil) is heavier than kerosene and has a higher boiling point (250 to 350 degrees Celsius). Each is optimized for specific applications.
3. Why was kerosene used more for lighting and heating in the past?
In the past, before the widespread expansion of electricity and natural gas networks, kerosene was a cost-effective and readily available option for lighting (kerosene lamps) and heating (kerosene heaters) in homes due to its easy combustibility and ability to produce suitable light and heat.
4. What is the most important current use of kerosene?
The most important current use of kerosene is as Jet Fuel in aircraft. This type of fuel must have specific characteristics, including a suitable flash point and stability at low temperatures, which kerosene possesses.
5. What role does hydrotreating play in kerosene production?
Hydrotreating is a purification process where kerosene reacts with hydrogen under high pressure and temperature in the presence of a catalyst to remove impurities such as sulfur compounds. This process improves kerosene’s quality in terms of color, odor, and stability, making it suitable for final uses.












I am now not positive the place you are getting your information, however great topic. I needs to spend some time studying much more or figuring out more. Thank you for wonderful info I used to be searching for this info for my mission.
Is the kerosene used in home heaters the same as the fuel used for commercial aircraft (Jet A-1)?
No, although both are kerosene-based, jet fuel undergoes much more rigorous refining processes. Specific additives are also included to prevent it from freezing at high altitudes and to maintain its stability.
Why is the removal of sulfur compounds (Hydrotreating) so important in the kerosene production process? Does it also affect the fuel’s odor?
Sulfur removal is not only essential to prevent equipment corrosion and reduce environmental pollution, but it also directly reduces the pungent odor of kerosene. This is particularly crucial for indoor domestic applications and kerosene heaters.