The Intricate Process of Gasoline Production | From Crude to Gasoline
Gasoline, a vital fuel composed primarily of hydrocarbons, is predominantly derived from crude oil. Its journey from an underground reservoir to your vehicle’s fuel tank is a complex process, involving numerous stages, each critical to the final product’s quality and characteristics. Broadly, this transformation can be broken down into several key phases:
Crude Oil Extraction: The initial step involves extracting crude oil from subterranean reservoirs. Crude oil itself is a heterogeneous mixture of hydrocarbons with varying carbon chain lengths.
Transportation and Storage: Once extracted, the crude oil is transported to refineries and stored in large tanks, awaiting processing.
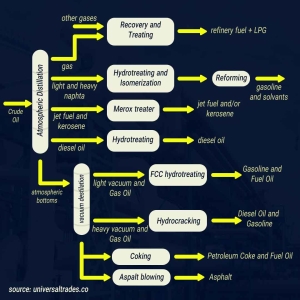
Crude Oil Distillation: This phase is arguably the heart of the refining process. Within the distillation unit, crude oil is heated and separated into different components, known as “fractions,” based on their boiling points. Gasoline, being one of the lighter fractions, boils at a lower temperature. Other fractions include refinery gases, naphtha, kerosene, diesel, fuel oil, and asphalt.
Naphtha Processing: The naphtha fraction is the primary feedstock for gasoline production. However, raw naphtha alone doesn’t meet the quality standards for gasoline and must undergo further processing.
Conversion Processes: To enhance quality and produce high-octane gasoline, naphtha and other heavier fractions are subjected to various conversion processes. The most significant of these include:
- Cracking: This process breaks down larger hydrocarbon molecules into smaller, more desirable ones. Cracking can be thermal (using heat) or catalytic (using catalysts). Fluid Catalytic Cracking (FCC) is a prevalent method for maximizing gasoline yield from heavier fractions.
- Reforming: This process rearranges the molecular structure of hydrocarbons, converting them into branched-chain isomers. Branched molecules possess a higher octane number. Catalytic Reforming is widely used to boost gasoline’s octane rating.
- Alkylation: In this reaction, smaller hydrocarbons (typically isobutane and alkenes) are combined to form larger, high-octane molecules.
- Isomerization: This process transforms straight-chain normal paraffins into branched-chain isoparaffins, which exhibit higher octane numbers.
Treatment and Blending: Following conversion, the gasoline components often contain impurities like sulfur compounds, which must be removed. To achieve the desired properties and octane rating, various gasoline components are then blended together. Additives such as detergents, corrosion inhibitors, and antioxidants are also incorporated at this stage.
Understanding Octane Number and Its Significance
The octane number is a crucial metric that measures gasoline’s resistance to premature ignition, often referred to as “engine knocking” or “pinging,” in internal combustion engines. A higher octane number indicates greater resistance to knocking, leading to improved engine performance. Engine knocking can result in reduced power, increased fuel consumption, and potential damage to engine components.
How Octane Levels Influence Gasoline Production
Producing gasoline with different octane ratings involves variations in the type and intensity of the conversion processes:
- Regular Gasoline (Lower Octane): The production of this gasoline type requires less intensive conversion processes. It might utilize lower-quality naphtha fractions and less severe cracking and reforming. This gasoline typically has a higher proportion of straight-chain hydrocarbons (normal paraffins).
- Premium/Super Gasoline (Higher Octane): Producing high-octane gasoline necessitates more extensive use of conversion processes such as reforming, alkylation, and isomerization. The primary goal is to increase the ratio of branched-chain hydrocarbons (isoparaffins) and aromatics, both of which possess high octane ratings. These processes are generally more complex and costly.
- Additives: Octane-boosting additives can also be used to raise gasoline’s octane number. Examples include MTBE (methyl tert-butyl ether) and ethanol. However, the use of some of these additives has been restricted due to environmental concerns.
- Super Plus Gasoline (Even Higher Octane): Some refineries produce gasoline with even higher octane numbers (e.g., “super plus” grades). These fuels are suitable for vehicles with advanced engines and high compression ratios that demand greater knock resistance. Their production involves maximizing the use of octane-enhancing processes and a more selective choice of naphtha fractions.
Conclusion
Gasoline production is a sophisticated and technologically advanced process, encompassing multiple stages and intricate chemical transformations. Understanding this journey, from crude oil extraction to refining and the creation of final products with varying octane ratings, holds significant importance in the oil and gas industry. Differences in gasoline octane directly correlate with variations in the type and intensity of refinery conversion processes, profoundly impacting vehicle engine performance and longevity.
Frequently Asked Questions (FAQ) About Gasoline Production
1. What exactly does “octane number” mean for gasoline?
The octane number of gasoline is a measure of its resistance to pre-ignition or knocking in internal combustion engines. Pre-ignition occurs when the fuel-air mixture spontaneously ignites due to high pressure and temperature in the cylinder, before the spark plug fires. This phenomenon causes a distinct “pinging” sound in the engine and can degrade performance. A higher octane number signifies greater resistance to pre-ignition.
2. Is using higher-octane gasoline beneficial for every car?
No, using higher-octane gasoline for vehicles that don’t require it typically offers no particular benefit. Your car’s owner’s manual is the best resource for determining the appropriate fuel type. Modern engines are designed to perform optimally with the manufacturer-recommended gasoline. Using a higher octane than necessary will only result in unnecessary additional cost.
3. What causes the price difference between different octane gasolines?
The price difference between gasolines with varying octane ratings stems from the increased complexity and cost associated with producing higher-octane fuel. To boost the octane number, refineries must employ more advanced chemical processes like reforming, alkylation, and isomerization, which demand more energy and often more expensive catalysts. Additionally, specific additives might be required to achieve higher octane levels, further contributing to the cost.
4. Can gasoline additives increase octane number?
Yes, some gasoline additives are specifically designed to increase the octane number. For instance, additives such as MTBE (methyl tert-butyl ether) and ethanol have been used in the past and present to raise octane. However, environmental concerns have led to restrictions on the use of some of these additives. It’s important to note that not all gasoline additives are capable of increasing octane; some are merely for cleaning the fuel system or improving performance under specific conditions.
5. What happens if I use gasoline with a lower octane than recommended?
Consistently using gasoline with a lower octane than recommended for your vehicle can lead to engine knocking. Knocking can result in reduced power and acceleration, increased fuel consumption, and in the long run, damage to internal engine components like pistons and valves. While modern vehicle engine control systems may attempt to compensate by adjusting ignition timing, this solution has limitations, and the best practice is to use the appropriate gasoline.












I haven’t checked in here for a while since I thought it was getting boring, but the last few posts are good quality so I guess I will add you back to my daily bloglist. You deserve it my friend :)
What are the most critical Conversion Processes in refineries that enhance gasoline quality?
The most critical processes that significantly increase gasoline quality and octane number are: 1. Catalytic Cracking to break down heavier molecules, 2. Catalytic Reforming to restructure chain hydrocarbons into high-octane aromatics, 3. Alkylation to combine small hydrocarbons into larger, high-octane molecules, and 4. Isomerization to convert straight-chain molecules into high-octane branched molecules.
Is the engine knocking sound on uphill roads always due to the low octane number of the gasoline?
Yes, in most cases, this sound indicates knocking or pre-ignition of the fuel. When the octane number is low, the fuel explodes before the spark plug fires, which can cause serious damage to the pistons in the long run.